|
Biological Magnetic Resonance Data BankA Repository for Data from NMR Spectroscopy on Proteins, Peptides, Nucleic Acids, and other Biomolecules |
Member of
|
BMRB Featured System: Lysozyme |
|||||
| |||||
|
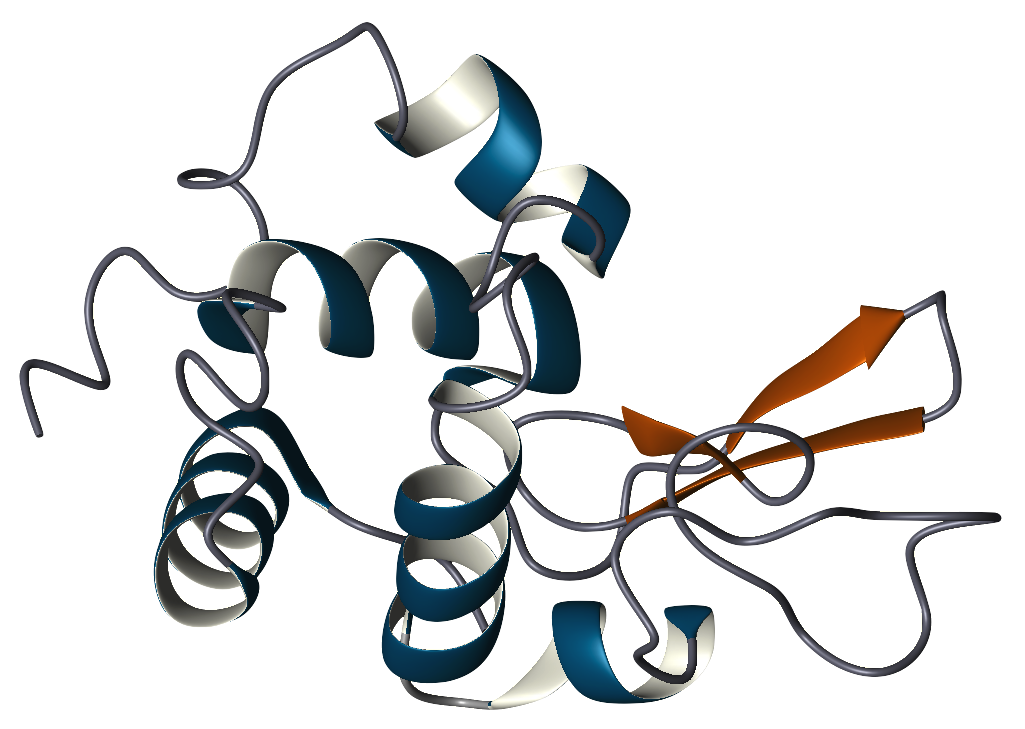
Lysozyme (1,4-β-N-acetylmuramidase) is an enzyme that plays an important role in the prevention of bacterial infections. It does this by attacking a specific component of certain bacterial cell walls, peptidoglycan. Peptidoglycan is composed of the repeating amino sugars, N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM), crosslinked by peptide bridges. Lysozyme acts by hydrolyzing the bond between NAG and NAM, increasing the bacteria's permeability and causing the bacteria to burst. Lysozyme is widely distributed in plants and animals. Human lysozyme is expressed in the mucous membranes of the nasal cavity and tear ducts. It is also found in saliva, tears, milk, cervical mucus, leukocytes, and kidney tissue. The majority of the lysozyme used in research is purified from hen egg whites.
The primary structure of lysozyme is a single polypeptide containing 129 amino acids. In physiological conditions, lysozyme is folded into a compact, globular structure with a long cleft in the protein surface. This cleft is the active site involved in binding to the bacterial carbohydrate chain and subsequently cleaving it. Lysozyme was discovered by Alexander Fleming in 1921 when he demonstrated that his own nasal mucus had the ability to inhibit the growth of a certain strain of bacteria in culture. He realized that this was largely due to the action of a protein within the mucus that caused the bacterial cells to lyse or break apart. Hence, he named the protein lysozyme. In a 1922 publication, he reported its activity in hens' egg white, tears, saliva, sputum, and nasal secretions. In a subsequent study, Fleming in collaboration with V. D. Allison detected lysozyme in human blood serum, saliva, milk, and a wide variety of other fluids. Despite lysozyme's antimicrobial activity toward harmless, airborne bacteria, it proved to be ineffective against disease causing bacteria. Fleming, realizing that there were no broad medical applications to his discovery, moved on to other studies in chemical antiseptics. However, his work on lysozyme further stimulated Fleming's interest in antimicrobial agents and consequently led to the discovery of penicillin in 1928, for which he later received a Nobel Prize in 1945. In 1966, David Chilton Phillips, using x-ray crystallography, determined lysozyme's structure, the first ever solved for an enzyme. From this work, Phillips was able to explain the mechanism of the enzyme's catalytic activity. Lysozyme is now one of the most abundant protein structures in the Protein Data Bank. Lysozyme was selected as the Protein Data Bank's Molecule of the Month in September of 2000. This page offers additional information on various Lysozyme-related topics, including history, structure, and function. | |||||
|
Next: Lysozyme Mechanism |